Safety in NETTER-2 was consistent with the established safety demonstrated in NETTER-11
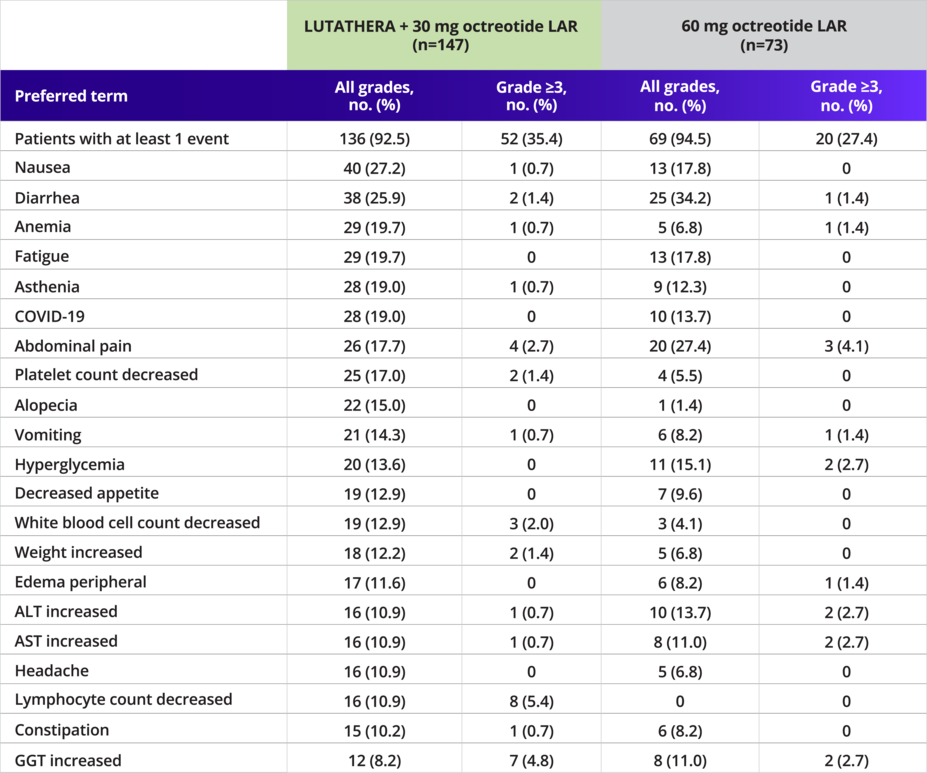
Adverse Events (Irrespective of Causality) by Preferred Term (≥10% Incidence in Either Arm) (Safety Set)2
The most common adverse events (≥20% in either arm) were nausea (27% vs 18%), diarrhea (26% vs 34%), and abdominal pain (18% vs 27%) for LUTATHERA + SSA vs SSA alone, respectively1
The most common grade 3/4 adverse events (>3% in either arm) were lymphocyte count decreased (5% vs 0%), GGT increased (5% vs 3%), small intestinal obstruction (3% vs 0%), and abdominal pain (3% vs 4%) for LUTATHERA + SSA vs SSA alone, respectively1
2% of patients needed a reduced dose and 5% discontinued treatment with LUTATHERA due to AEs1
No new safety signals were reported in NETTER-2
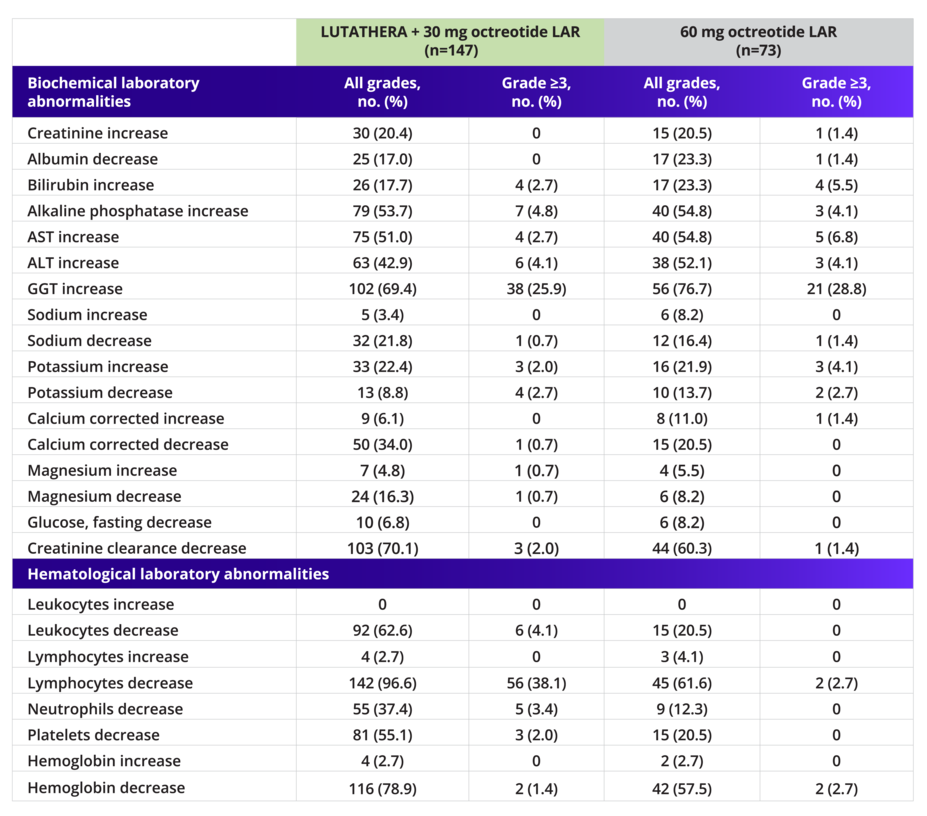
Incidence of Laboratory Abnormalities (Safety Set)3
Incidence based on worst post-baseline CTC grade.
1L, first line; AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTC, Common Terminology Criteria; GGT, gamma-glutamyl transferase; LAR, long-acting release; MDS, myelodysplastic syndrome; SSAs, somatostatin analogues; SSTR+, somatostatin receptor-positive.