Mechanism of Action
Overview
LUTATHERA Is the First FDA-Approved RLT for the Treatment of Adult Patients With SSTR-Positive GEP-NETs1
LUTATHERA |
Somatostatin Receptor Expression
GEP-NETs Overexpress SSTR Subtype 22
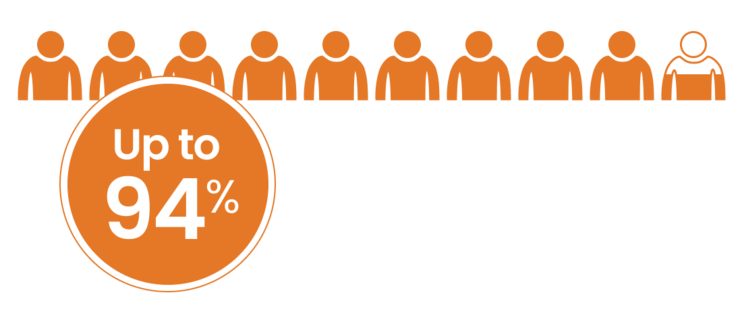
of GEP-NETs have been demonstrated to express SSTRs, with 86% expressing SSTR subtype 22,a,b
aBased on a study of 100 cases with clinical and pathological data selected from a prospectively built database of patients with gastroenteropancreatic endocrine tumors referred from 3 institutions.2
bThis work was supported by grants from Novartis.
Patients with GEP-NETs with a high density of SSTR expression may be considered for targeted treatment with LUTATHERA.3
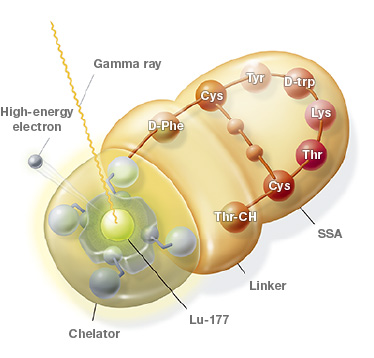
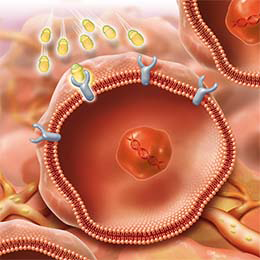
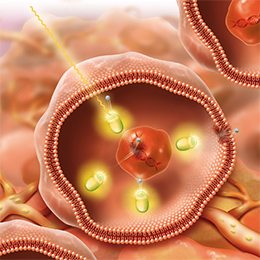
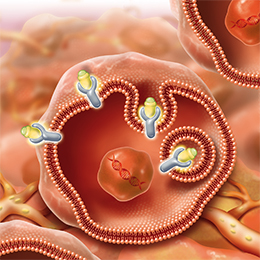
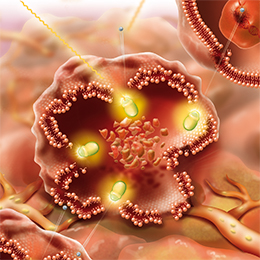
Mechanism of Action for LUTATHERA
LUTATHERA is a targeted treatment that uses radiation to damage SSTR-positive cells and neighboring cells.3
PRECISION TARGETING FOR | ENTERS THE CELLS3 |
|
|
|
|
FDA, US Food and Drug Administration; GEP-NETs, gastroenteropancreatic neuroendocrine tumors; RLT, radioligand therapy; SSA, somatostatin analog; SSTR, somatostatin receptor.