The dosing for LUTATHERA remains the same, regardless of patient type1
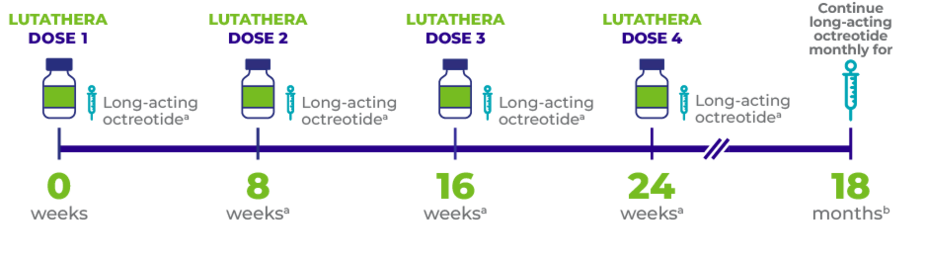
Recommended Treatment Regimen With LUTATHERA1
aAdminister long-acting octreotide 30 mg IM between 4 to 24 hours after each dose of LUTATHERA. Do not administer long-acting octreotide within 4 weeks prior to each subsequent dose of LUTATHERA. The interval between infusions may be extended up to 16 weeks in the case of a dose modification due to an adverse reaction. Permanently discontinue LUTATHERA in patients who experience grade 3/4 hypersensitivity reactions. Please see the Prescribing Information for additional information on dose modifications.1
bContinue long-acting octreotide 30 mg IM every 4 weeks after completing LUTATHERA until disease progression or for 18 months following treatment initiation at the discretion of the physician.1
During treatment, administer long-acting octreotide 30 mg IM between 4 to 24 hours after each dose of LUTATHERA
LUTATHERA dosage should be modified based on hematologic, renal, hepatic, hypersensitivity, or other adverse reactions (see full Prescribing Information)1
For reduced dose administration instructions, refer to section 2.5 (Preparation and Administration) of the full Prescribing Information1
The recommended dose for LUTATHERA is 7.4 GBq (200 mCi) every 8 weeks (±1 week) for a total of 4 doses1
Before each dose of LUTATHERA1
DO NOT ADMINISTER LONG-ACTING SSAs FOR AT LEAST 4 WEEKS
DO NOT ADMINISTER SHORT-ACTING SSAs FOR AT LEAST 24 HOURS
88% of patients completed all 4 doses of LUTATHERA in NETTER-2 and 77% of patients completed all 4 doses of LUTATHERA in NETTER-11
Dose Modifications
Recommended dose modifications of LUTATHERA for adverse reactions1
†Including allergic reaction and anaphylaxis.1
‡No dose modification required for hematological toxicities of grade 3 or grade 4 solely due to lymphopenia.1
Preparing and Administering LUTATHERA
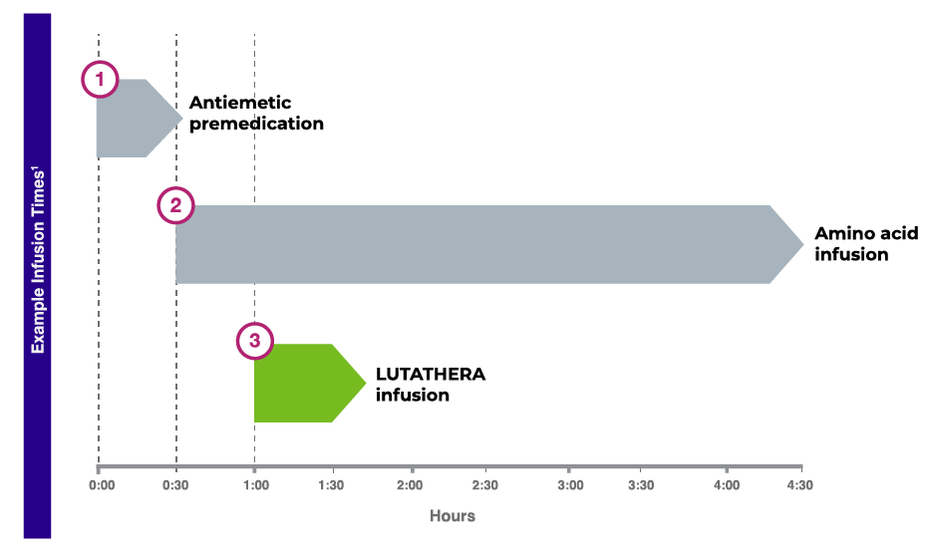
Infusion schedule and procedures
Treatment with LUTATHERA also includes premedications and amino acid infusion.1
Hypersensitivity prophylaxis: Premedicate patients who have had prior grade 1/2 hypersensitivity reactions to LUTATHERA. Do not rechallenge patients who experience grade 3/4 hypersensitivity reactions to LUTATHERA.1
Refer to the LUTATHERA Prescribing Information for more detailed information on radiation requirements and patient counseling